Biological samples transportation …
The ADR regulations – Accord for Dangerous Goods by Road – manage the biological samples transportation.
As soon as the biological samples are on the public domain, the ADR regulation applies
SAMPLES
All biological samples are covered and must respect the ADR: FALSE!
The specimens concerned by this regulation are all biological products, substances or materials derived from living organisms; that is, bacteria, fungi, viruses, animals or humans). And these samples are extracted or purified for the purpose of being used for preventive, therapeutic or diagnostic uses.
Mostly, this will involve urine, blood and swab samples.
It is important to note that there is an exemption list. This list is based on the fact that the sample being transported does not pose a health risk.
Therefore, ADR does not involve many samples:
– Stool samples for blood testing
– Blood products for transfusion
– Soil and water samples sent for research that are considered safe for human and animal infection.
– …
Check the exemptions list on the WHO website
Exempted biological samples are not regulated for transport: WRONG!
Biological samples from humans or animals, exempted from the ADR, must however be packed in a triple packaging according to the same characteristics as a classical biological sample.
The difference concerns the outer packaging labelling, which must mention the words ” Exempted sample of human origin ” or ” Exempted sample of animal origin “.
TRIPLE PACKAGING
Exempted biological samples are not subject to transport regulations: FALSE!
The ADR classifies samples according to the infectious substances’ origin: A or B class
Each class has its own ADR packing instructions: P620 and P650.
A UN number is issued to indicate the infection risk level:
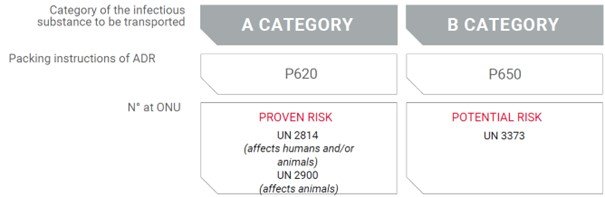
Extract from the LABELIANS documentation
For category B infectious substances, 3 levels of packaging must be used, each of which must comply with specific and REQUIRED characteristics:
- Level 1 = primary packaging
- This is a container, a collection tube
- This packaging must be leakproof
- Level 2 = secondary packaging
- This is a pocket or a box with an absorbent solution to absorb 100% of the sample transported
- This container must also be imperatively leakproof
![]() Besides, 1 of the 2 packages (primary or secondary) must be resistant to an internal pressure of 95kPa.
Besides, 1 of the 2 packages (primary or secondary) must be resistant to an internal pressure of 95kPa.
- Level 3 = outer packaging
- This is a bag, a cooler, a case…
- This packaging must be resistant and have a minimum surface of 100x100mm to allow regulatory labelling

The compliance report is not required for this configuration.
This means that each unit can come from different brands.
However, the user must ensure that the transport configuration is suitable to withstand a 1.2m drop.
Compliant transport configuration for Category B infectious substances:

Biological substance category B
For Category A infectious substances, 3 packaging levels should be used, also with the same technical characteristics as above.
The main difference is the regulatory labeling that must correspond to Category A infectious materials. 
To comply with the regulations, complete kit solutions are considered the right choice.
Compliant transport configuration for Category A infectious substances:

Biological substance category A
Regulations limit the infectious materials amount that can be transported: RIGHT!
For category B (UN3373), for air shipments, the primary container must not contain more than 1 liter and the maximum quantity for the outer packaging is 4L (excluding cold source)
For road, rail or sea transport, there is no quantitative limit.
For category A (UN2814 or UN2900), for air shipments, the maximum quantity of the primary container is:
– 50ml or g if transported by air with passengers
– 4L or 4kg if transported by cargo aircraft
As for category B, transport, by road, rail or sea, does not require any quantitative limit for category A infectious substances.
LABELIANS experts are available to support you in the choice of your transport solution in compliance with the ADR; that’s RIGHT !