How to guarantee samples conformity before analysis? Sample compliance when received in the laboratory is imperative to guarantee the analyticial results accuracy.
This compliance is totally linked to the sampling and transport steps.
Perfect procedures and standards management is necessary in every pre-analyticial process phase.
Sample is collected!
Biological sample must be collected in a suitable container that complies with its collection; but also complies with the biological samples transportation.
This is because biological samples are considered as dangerous goods. Its packaging must comply with standards in order to be placed on the public domain: these are the packaging instructions.
How to choose the right transport solution?
Transport solution must be adapted to the sample collected. This must guarantee the sample integrity for analysis.
It is also important to adapt the transport solution to the samples volume. However it is also important that bags are not too heavy or too large for the courrier.
Transport may also requires temperature control at a precise value; this is temperature-controlled transport.
In the case of temperature-controlled transport, it is imperative to select coolers with good thermal benefits or a transport solution in a mobile thermostatic chamber, either autonomous (battery-powered) or connected to vehicles.
The choice between a high-performance bag or an electronic transport box is very closely linked to the available budget, the frequency of use. But the major parameter is the transport duration while the sample must imperatively be maintained at the right temperature.
What are the packaging instructions?
The majority of biological specimen shipments involve Category B specimens; that is, specimens that do not cause death or permanent disability.
UN number UN3373 identifies samples and the packing instructions are called P650.
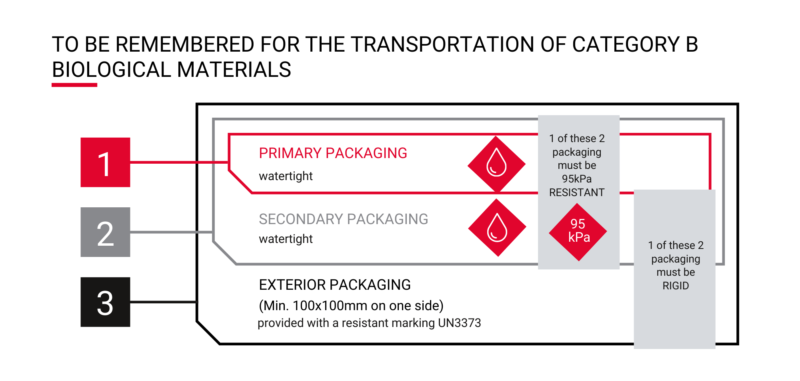
This means that to transport blood, urine, stool, cytological samples, etc., the transport solution must includes 3 packages (triple packaging) consisting of :
1- A primary leak proof packaging and if possible resistant to a pressure of 95kPa
- This is the case for all blood collection tubes!
2- A secondary packaging that is leakproof and resistant to a pressure of 95kPa if the primary packaging is not
- For the transport of a urine bottle that is not 95kPa resistant, it will be required to use a 95kPa resistant secondary packaging such as a double bag to isolate the documents provided
3- A rigid outer packaging, if the secondary packaging is not rigid, and sufficiently resistant with a size that allows the regulatory information to be marked, i.e. an area of 100x100mm.
- For a 95kPa bag with a urine bottle, a rigid cooler should be chosen
- For blood samples packed in a secondary airtight packaging such as a Lock&Lock box, the flexible bag will be perfectly adapted
LABELIANS supports its customers in their transport solution elaboration with tested and validated products in usual transport conditions.
Test results are available to provide our customers with the ability to validate their own transport conditions in compliance with Cofrac accreditation.