It is crucial to be well supported towards the new IVD regulation:
- To fully understand the issues and their impact on daily consumables
- To be serene in maintaining the COFRAC certification of the laboratory
- To be able to make better consumables supplier choices, today and tomorrow.
Regulation 2017/746 (IVDR) – REGULATION (EU) 2017/746 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU– is evolving with a first milestone of 26 May 2022 for non-sterile Class A products.
The biology you are interested in is medical. That means you have to qualify many contraints to meet services quality expected levels:
- Patients welcoming process, from their identity mastering to their medical background…
- Teams training
- Risk coming along samples transportation mitigation
- Professional risks assessment : blood exposure accidents, musculoskeletal disorders, contaminations…
- Proper equipment maintenance and performance checking
- Right labs consumables and other materials qualification
- Special attention to critical consummables
When a change affects this last topic, you need to get support and assistance from partners who can help you remaining able to be a biology and anatomopathology laboratory that provides patients :
- A reproducible and reliable diagnostic
- in the shortest delivery time
- and at an optimized economical cost.
Being supported to well understand challenges and impacts on your daily routine consumables
Laboratory supplies covered by the regulation are described as “any medical device that consists of a reagent, […] an instrument, […] intended by the manufacturer to be used in vitro in the examination of specimens from the human body, […] for the purpose of providing information on one or more of the following :
- about a proces or a specific pathology or physiological condition
- linked to congenital physical or mental deficiencies
- proneness to a condition or disease
- to determine whether a given treatment is safe for and compatible with potential recipients
- predicting response or reactions to a treatment
- to define or control therapeutic measures
Specimen containers are also deemed to be in vitro diagnostic (IVD) medical devices.”
The IVDR does not apply to:
“(a) products intended for general laboratory use or products intended exclusively for research, unless, having regard to their characteristics, such products are specifically intended by their manufacturer for in vitro diagnostic examinations;
“Products that do not have a specific characteristic and are intended for in vitro diagnostic tests are not IVDs
(b) invasive products intended for the collection of samples or products placed in direct contact with the human body for the purpose of obtaining a sample;”
“Needles and other sampling materials are affected by the MDR: Medical Device Regulations (2017/745)
IVD are classified in 4 main classes depending on which functionnality each is aimed, and risks attached to their use.
98/79/CE directive made no difference; one product was IVD or was not, without any other restriction.
While the regulation presents subcategories called classes. These classes have been established according to their purpose and the risks associated with their use; from the lowest risk product (Class A) to the highest risk product (Class D).
- Class A: low public health risk and low individual patient risk
Example: specimen containers, instruments - Class B: low public health risk and/or moderate individual patient risk
Example: some self-tests, controls - Class C: moderate public health risk and/or high individual patient risk
Example: cancer markers, genetic tests, companion tests, flu tests - Class D: high public health risk and high patient risk
Example: blood type, transmissible agents, HIV testing
IVDR will strongly impact in vitro diagnostic devices sector.
- Today 80 to 85% of IVD are auto-certified
- Go-to-market certification is run 100% by the manufacturer
- No preliminary validation or screening via independant control is demanded – especially regarding claimed benefits-.
- With the regulation, 90% of IVD roughly will be submitted to notified control instance evaluation: all of class B to D devices and sterile class A devices .
- This will consequently lead to reinforcd control of manufacturers claims about benefits and risks for the patients.
- This will, in parrallel lead to extended development and go-to-market processes for new products programs.
Being well supported to antcipate the IVD R progressive roll out
You may need to have at arms length all documents you need to update your products files.
Ask your lab supplies supplier to get them. he mus have indeed worked far before regulatory changes on a forecats plan to help you through the process.
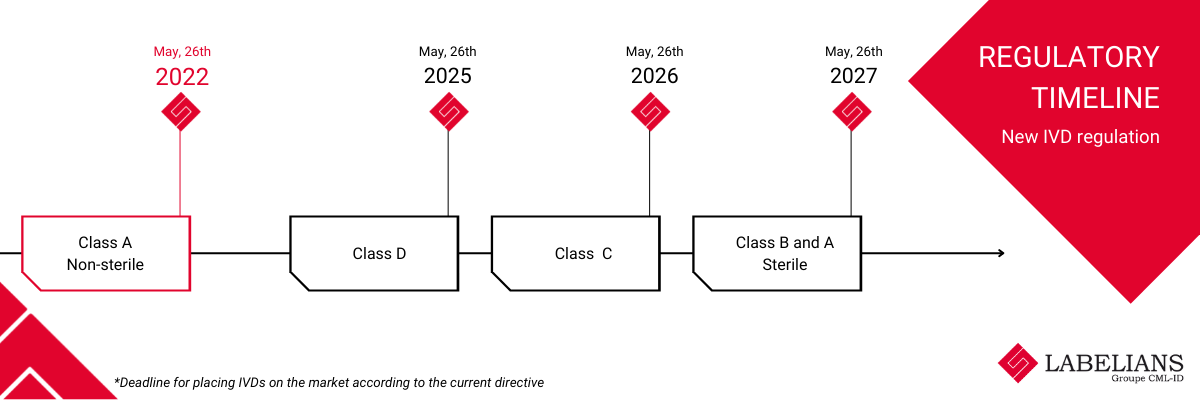
What you should expect from your supplier right before the upcoming frist phase.
On 2022, May 26th, your lab supplier must have identified supplies that fall under IVD Regulation .
That means technical specs sheets are updated and available on request, for any product you may need to buy.
Products that are historically identified as IVD as per the directive can still carry this labeling with the new IVD regulation or will simply become General Laboratory Usage producst and won’t be regulated anymore.
Vous devez être accompagné aussi après le 26 Mai !
Ce changement va vous proposer un nouveau regard sur les choix de consommables, ou de matériels que vous aller effectuer pour votre laboratoire. Plus que jamais, parce qu’il s’agit d’un changement que vous devez maitriser pour être rassuré sur votre capacité à maintenir l’accréditation COFRAC de votre laboratoire, vous devez choisir un fournisseur de consommables et matériels pour le laboratoire qui :
- Garantit que son offre fait l’objet d’un contrôle rigoureux auprès de ses partenaires fabricants afin de vous garantir la mise à disposition de produits conformes au nouveau règlement.
- Met en place un contrôle des documents qualité sur l’ensemble des produits concernés par le règlement du 27 octobre 1998 relative aux dispositifs médicaux de diagnostic in vitro.
- Assure une communication systématiquement en cas d’évolution des produits et/ou de mise à jour des fiches techniques, et qui partage cet engagement avec vous sur simple demande.
- Assure la traçabilité des produits distribués (n° lot)
- Assure la réacto-matériovigilance des produits concernés et dispose d’une procédure disponible sur simple demande.
- Vérifie l’existence de déclarations CE de conformité pour les produits concernés et vous la transmet sur simple demande
- A mis en place un processus de traitement des réclamations qu’il met à votre disposition sur simple demande
You need to be supported ALSO after May 26th !
This change is likely to bring a new perspective on the consumable choices or materials you will make for your laboratory. More than ever, because it is a change that you must master to be reassured on your capacity to maintain the COFRAC accreditation of your laboratory, you must choose a supplier of consumables and materials for the laboratory that :
- Guarantees that its offer is subject to rigorous control by its manufacturing partners in order to ensure the availability of products that comply with the new regulation.
- Implements a control of the quality documents on all the products concerned by the DIRECTIVE 98/79/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of October 27, 1998 on in vitro diagnostic medical devices.
- Ensures a systematic communication in case of product evolution and/or data sheet update, and shares this commitment with you upon request.
- Ensures the traceability of distributed products (batch number)
- Ensures the reacto-materiovigilance of the products concerned and is the subject of a procedure available on request.
- Checks the existence of EC declarations of conformity for the products concerned and sends them to you on request
- Has set up a process for handling complaints which it makes available to you on request
Be supported to benefit from a better quality of use thanks to the IVD regulation
Currently, many consumables (except those specific to certain strict uses) are self-declared by manufacturers as DM or IVD.
There is therefore no burden of proof for the manufacturer, and safety may therefore sometimes be insufficient for medical laboratories. Finally, the prequalification of these products is uncertain in the context of medical diagnosis.
Tomorrow, the manufacturer will have a much greater burden of proof:
There will be more performance studies before any products are placed on the market: the manufacturer will have to provide factual evidence. Both regulations imply the implementation of a real risk management approach throughout the life of the devices. In addition, manufacturers who display a CE mark on their products will have to strengthen their quality system and their risk management system.
You will still be able to use a DIV DM that has become a UGL for the same purpose as before
As today, you will qualify the device and take responsibility for its use in your medical laboratory
In conclusion, in this change, the role of the distributor or manufacturer becomes crucial.
The selection by distributors and the relevance of the selection criteria will be a guarantee of safety for medical laboratories. The ability of manufacturers to justify their CE marking will be a greater guarantee than self-declaration.
Choosing the right partner to benefit from the best support towards the IVD R is a collaborative issue that also allows to rethink certain supplies choices.
We, of course, recommend LABELIANS because for 50 years, it has been the preferred manufacturer and distributor of medical biology and anatomopathology laboratories, precisely for the regulatory quality of its offer and the support it provides through LABELIANS smart service to laboratories.
NEED HELP ?